Influenza A (H1N1) 2009 Monovalent Vaccine
- 14 pages
- September 2009
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Influenza A (H1N1) 2009 Monovalent Vaccine safely and effectively. See full prescribing information for Influenza A (H1N1) 2009 Monovalent Vaccine.
Influenza A (H1N1) 2009 Monovalent Vaccine
Manufactured by CSL Limited
Suspension for Intramuscular Injection
Initial U.S. Approval: 2007…
…
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome (GBS)
If GBS has occurred within 6 weeks of previous influenza vaccination, the decision to give Influenza A (H1N1) 2009 Monovalent Vaccine should be based on careful consideration of the potential benefits and risks.
5.2 Altered Immunocompetence
If Influenza A (H1N1) 2009 Monovalent Vaccine is administered to immunocompromised persons, including those receiving immunosuppressive therapy, the immune response may be diminished.
5.3 Preventing and Managing Allergic Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine.
5.4 Limitations of Vaccine Effectiveness
Vaccination with Influenza A (H1N1) 2009 Monovalent Vaccine may not protect all individuals.
6 ADVERSE REACTIONS
CSL’s Influenza A (H1N1) 2009 Monovalent Vaccine and seasonal trivalent Influenza Virus Vaccine (AFLURIA) are manufactured by the same process. The following sections summarize data obtained from clinical studies and postmarketing experience with AFLURIA.
6.1 Overall Adverse Reactions
Serious allergic reactions, including anaphylactic shock, have been observed during postmarketing surveillance in individuals receiving AFLURIA.
The most common local (injection-site) adverse reactions observed in clinical studies with AFLURIA were tenderness, pain, redness, and swelling. The most common systemic adverse reactions observed were headache, malaise, and muscle aches.
6.2 Safety Experience from Clinical Studies
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a vaccine cannot be directly compared to rates in the clinical studies of another vaccine and may not reflect the rates observed in clinical practice.
Clinical safety data for AFLURIA have been obtained in two clinical studies (see Clinical Studies [14]).
A US study (Study 1) included 1,357 subjects for safety analysis, ages 18 to less than 65 years, randomized to receive AFLURIA (1,089 subjects) or placebo (268 subjects). There were no deaths or serious adverse events reported in this study.
A UK study (Study 2) included 275 subjects, ages 65 years and older, randomized to receive preservative-free AFLURIA (206 subjects) or a European-licensed trivalent inactivated influenza vaccine as an active control (69 subjects). There were no deaths or serious adverse events reported in this study.
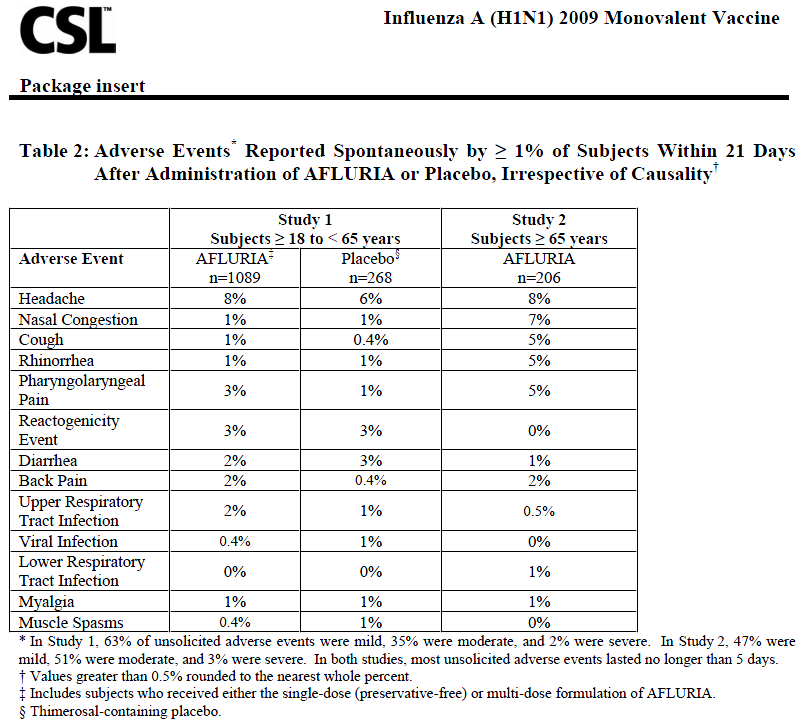
The safety assessment was identical for the two studies. Local (injection-site) and systemic adverse events were solicited by completion of a symptom diary card for 5 days post-vaccination (Table 1). Unsolicited local and systemic adverse events were collected for 21 days post-vaccination (Table 2). These unsolicited adverse events were reported either spontaneously or when subjects were questioned about any changes in their health post-vaccination. All adverse events are presented regardless of any treatment causality assigned by study investigators.
…
6.3 Postmarketing Experience
Because postmarketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. The adverse reactions described have been included in this section because they: 1) represent reactions that are known to occur following immunizations generally or influenza immunizations specifically; 2) are potentially serious; or 3) have been reported frequently.
The following adverse reactions also include those identified during postapproval use of AFLURIA outside the US since 1985.
Blood and lymphatic system disorders
Transient thrombocytopenia
Immune system disorders
Allergic reactions including anaphylactic shock and serum sickness Nervous system disorders
Neuralgia, paresthesia, and convulsions; encephalopathy, neuritis or neuropathy, transverse myelitis, and GBS
Vascular disorders
Vasculitis with transient renal involvement
Skin and subcutaneous tissue disorders
Pruritus, urticaria, and rash
General disorders and administration site conditions
Influenza-like illness (e.g., pyrexia, chills, headache, malaise, myalgia), injection-site inflammation (e.g., pain, erythema, swelling, warmth), and induration
6.4 Other Adverse Reactions Associated With Influenza Vaccination
Anaphylaxis has been reported after administration of AFLURIA. Although AFLURIA and Influenza A (H1N1) 2009 Monovalent Vaccine contain only a limited quantity of egg protein, this protein can induce immediate hypersensitivity reactions among persons who have severe egg allergy. Allergic reactions include hives, angioedema, allergic asthma, and systemic anaphylaxis (see Contraindications [4]).
The 1976 swine influenza vaccine was associated with an increased frequency of Guillain-Barré Syndrome (GBS). Evidence for a causal relation of GBS with subsequent vaccines prepared from other influenza viruses is unclear. If influenza vaccine does pose a risk, it is probably slightly more than one additional case per 1 million persons vaccinated.
Neurological disorders temporally associated with influenza vaccination, such as encephalopathy, optic neuritis/neuropathy, partial facial paralysis, and brachial plexus neuropathy, have been reported.
Microscopic polyangiitis (vasculitis) has been reported temporally associated with influenza vaccination.