Director’s Update Brief
- 37 pages
- Day 119
- August 14, 2009
– Key events:
– novel 2009-H1N1 Declarations
- WHO: Pandemic Phase 6 (11 JUN 2009 1600 EDT)
- Outbreaks in at least one country in > two WHO regions
- USG: Public Health Emergency declared (26 Apr 2009)
- Renewed by HHS Secretary Kathleen Sebelius
- HHS: Downgraded to Phase 1 – Awareness (9 May 2009)
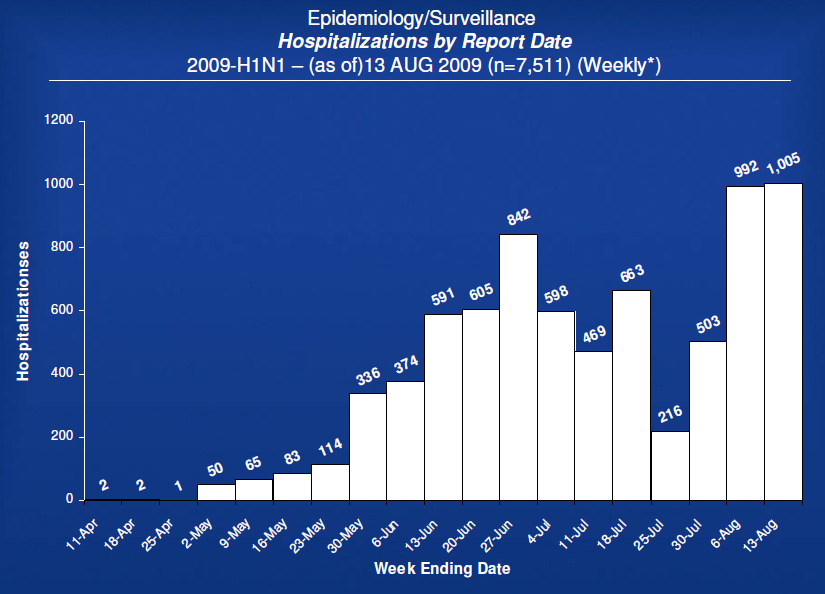
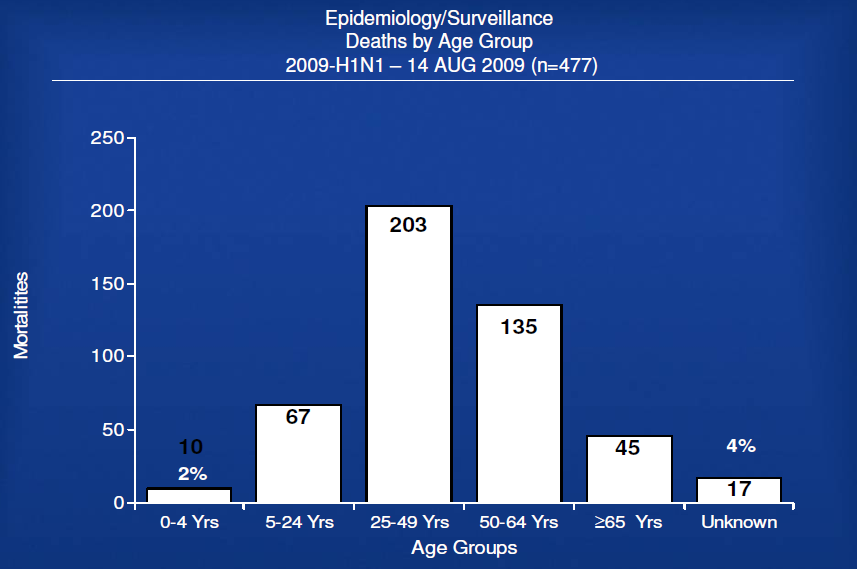
– US Cases (as of 13 Aug 2009; next update 21 August 2009)
– No longer reporting total cases.
- Centralized distribution by contractor receiving vaccine from 5 manufacturers and ancillary supplies from up to 4 manufacturers and shipping to sites (up to 80,000), under direction of state health departments
- System similar to VFC
- Vaccine record cards provided by CDC and included in the ancillary supply kit
- VIS posted on the CDC website
~August 18
– Posting of ACIP recommendations
– Family of related communication products and information, such as
- Additional description of program
- School-located vaccination guidance
- Provider information on enrollment
- Adverse Events drill: CDC, FDA, HHS—mid-Sept
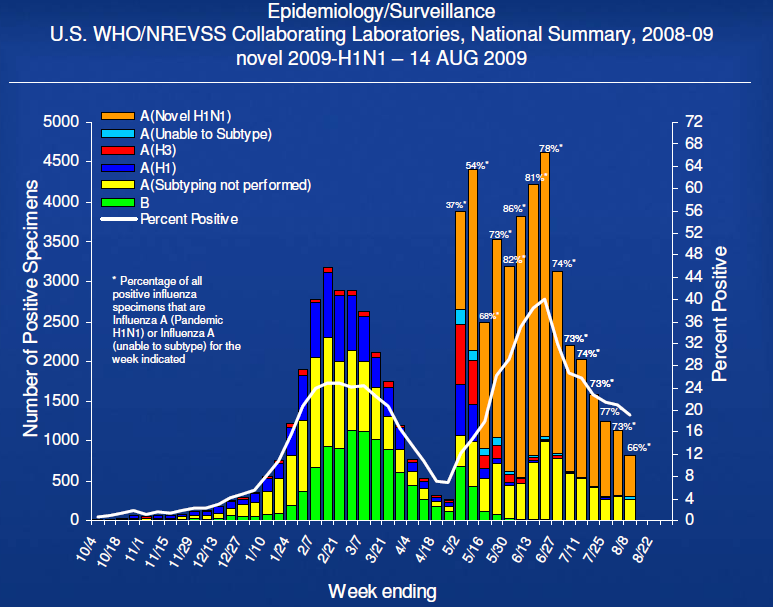
- Novel influenza A (H1N1) is now the predominate influenza subtype in circulation
– 66% of influenza viruses in the Northern Hemisphere
– 89% of influenza viruses in the Southern Hemisphere
- Epidemiology and clinical characteristics in Southern Hemisphere are similar to United States experience in spring
- No changes in the virus