National Center for Immunization and Respiratory Diseases
National Center for Immunization and Respiratory Diseases
- Dan Jernigan, MD MPH, Deputy Director, Influenza Division
- 25 pages
- For Official Use Only
- For Internal Use Only
- October 8, 2009
Detection of First Case
●Mesoscale device used to diagnose influenza in 10 year old boy during clinical trial run by Naval Health Research Center (NHRC) in San Diego on April 1, 2009
●Result is influenza A positive, however, H1, H3, H5 negative
●“Unsubtypable”confirmed by reference laboratory and by designated State Public Health Laboratory using FDA-cleared 5 Target PCRWisconsin
…
Response to H1N1
•Strategic National Stockpile
•Distributed 25% pro rata supply
•Enhanced Surveillance Initiated
•PCR panH1N1 kits for testing
•Development at CDC, EUA at FDA, manufacture at ATCC, and ready to ship in ~ 2.5 weeks
•Distributed Kits, so far:
•Domestic: 95 labs
•DOD: 15 labs
•International: >250 labs in 140 countries
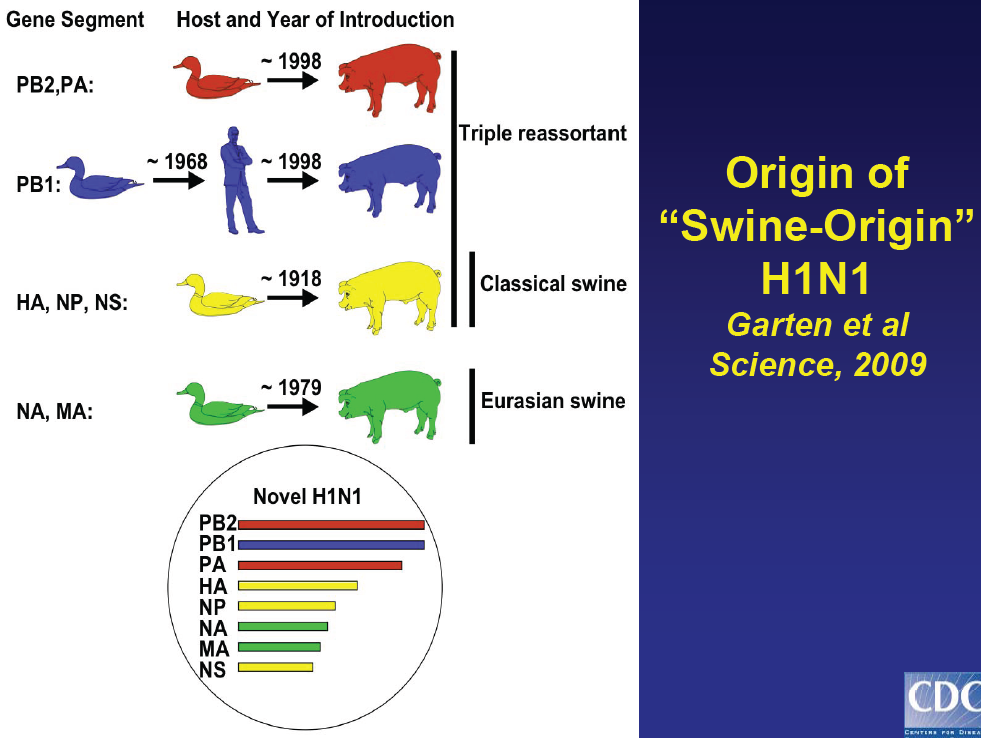
•Virus Characterization
•>1000 genes sequenced from >260 viruses
•Submitted to GenBankH1N1 Current Status
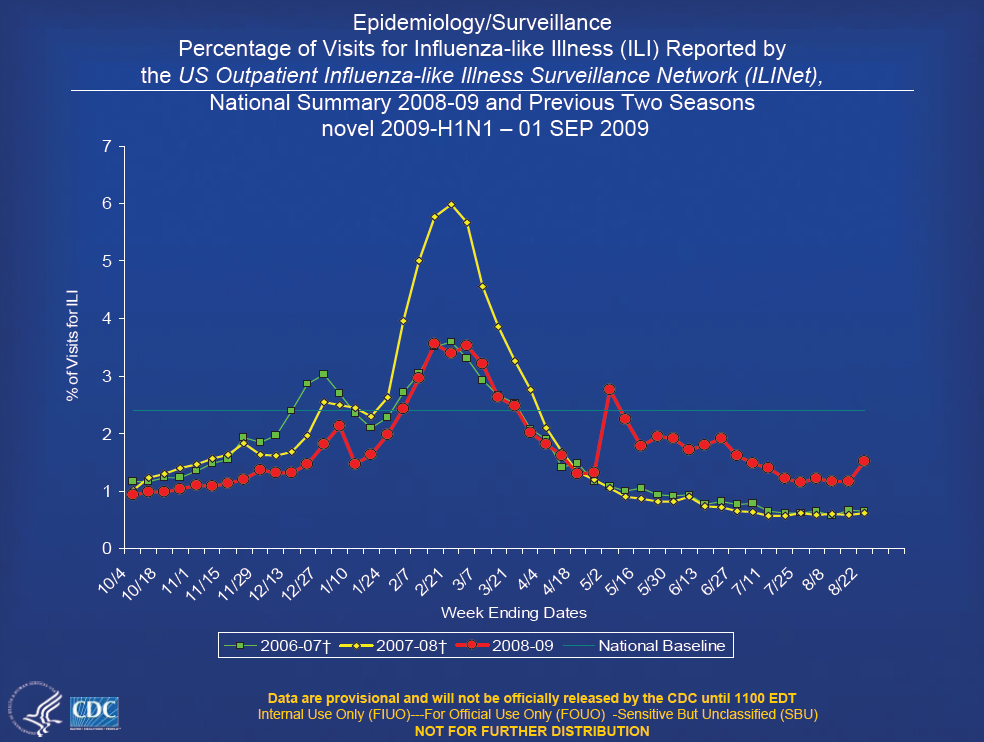
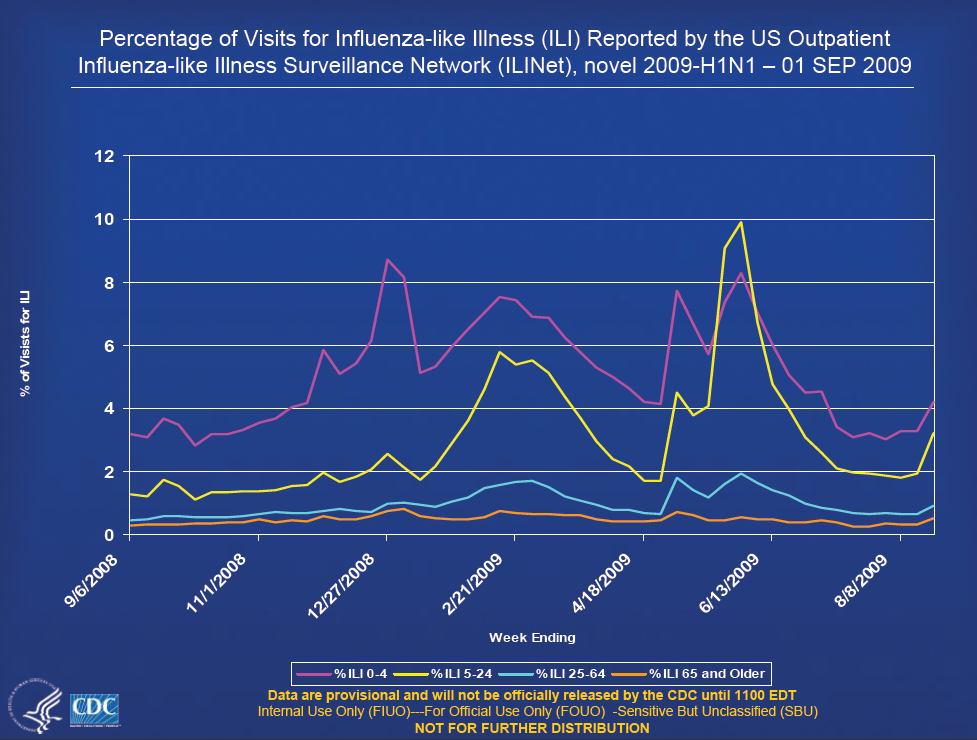
- Lab-Confirmed Cases
- 44,317 total cases when reporting stopped in July
- As of August 28, 2009
- 8,842 hospitalized
- 555 deaths
- Represents approximately 3 M cases
- Overall activity has declined since schools closed, but focal areas of activity have increased
- Viruses in US and Internationally show no evidence of significant genetic/antigenic change
…