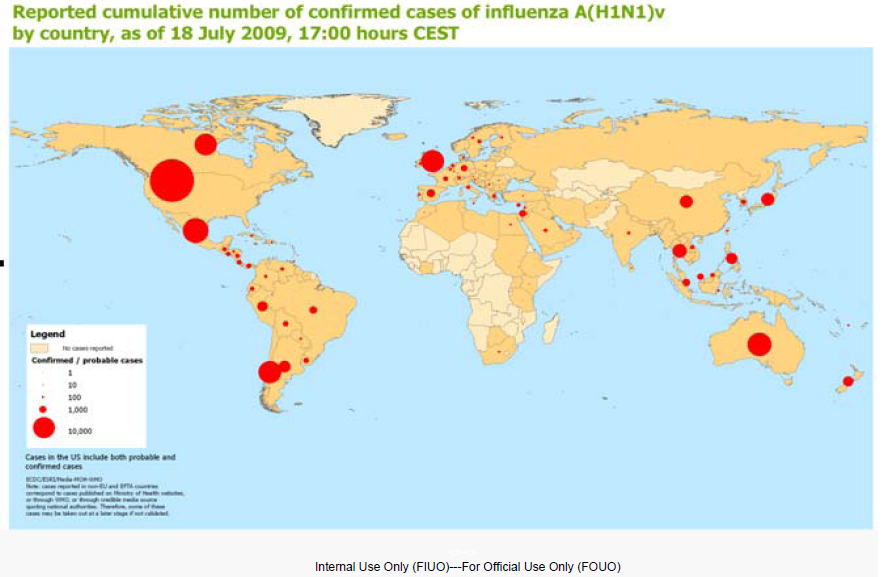
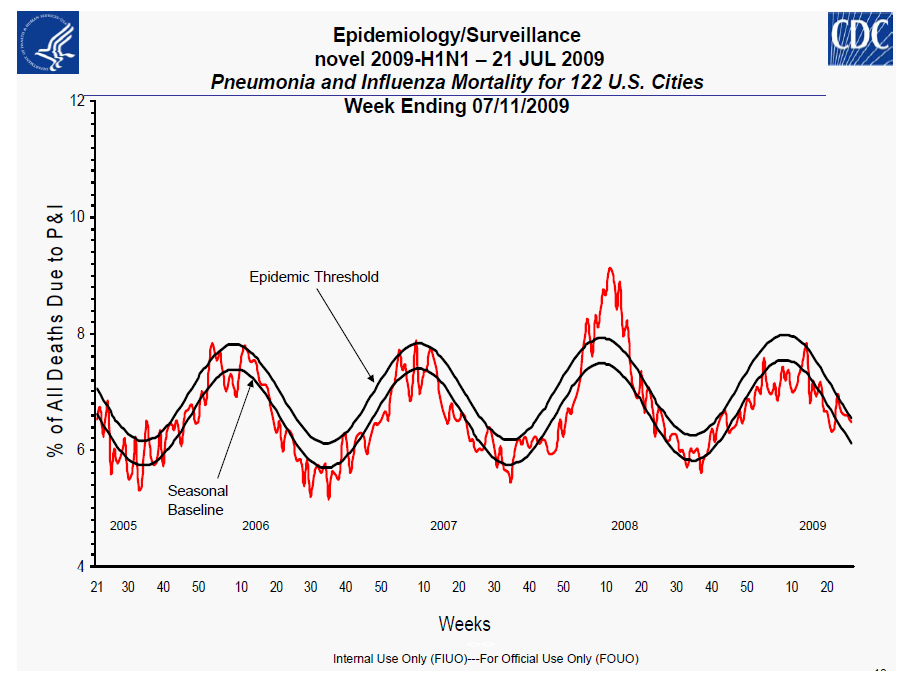
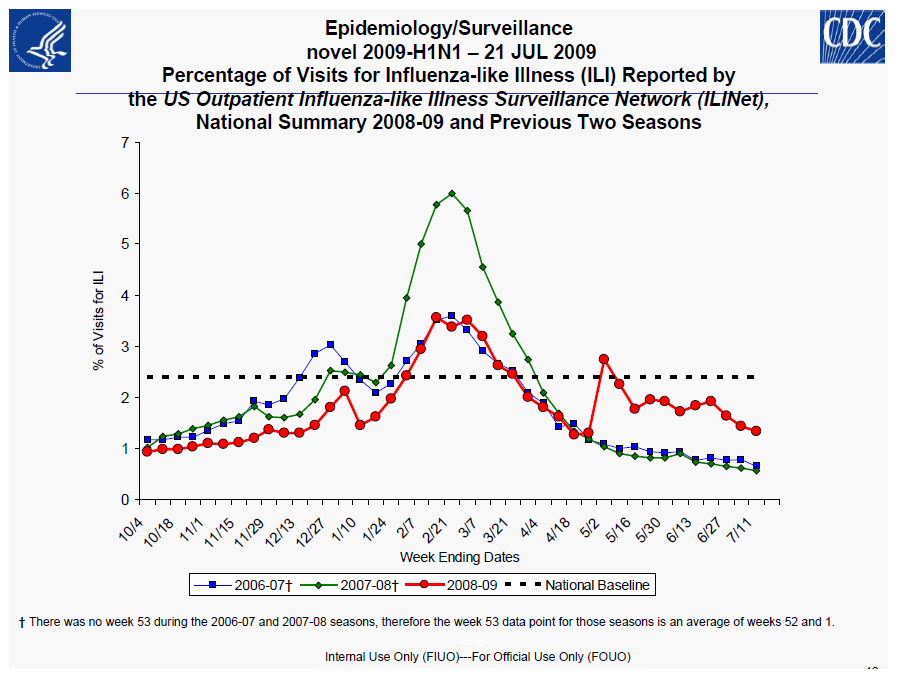
Centers for Disease Control
- 18 pages
- For Official Use Only
- For Internal Use Only
- July 21, 2009
– novel 2009-H1N1 Declarations
• WHO: Pandemic Phase 6(11 JUN 2009 1600 EDT)
• USG: Public Health Emergency declared (26 Apr 2009)
• HHS: Downgraded to Phase 1 Awareness (9 May 2009)Only hospitalizations and deaths of cases will be reported as of 23 July 2009.
…
• Conference call Friday 7/17 with Laboratory Partners’ Organizations regarding interpretation of Rapid Influenza Diagnostic Test results
-General agreement on guidelines
•Refined Guidance for RIDT Testing
— submitting final draft to CDC clearance emphasizing “use negative test results with caution”
•Possibly different testing algorithms for “peak” (high prevalence) and “off season” testing
-Suggest single document for clinicians and laboratories
-Suggest guidelines precede or accompany MMWR on RIDT performance data
• FDA Proposed Changes to EUA for novel 2009-H1N1 PCR
-In discussion w/FDA regarding new rule to limit PCR testing for novel 2009-H1N1 to only FDA-approved laboratories submitting validation via EUA
-Further engagement needed to determine it new rule may lead to increased testing at PHLs…
• 7/20/09 Tweets:
— Shock that Australia’s worst case scenario predicts 6,000 novel 2009-H1N1 deaths
— novel 2009-H1N1 tests are unreliable
— Thailand to step upgu screening measures
Hong Kong reports 6 new cases
Users say they avoid coworkers, friends, relatives, etc. who may have novel 2009-H1N1
— First confirmed novel 2009-H1N1 death on Guam
— “20,000 people die from novel 2009-H1N1 and everybody wants to wear a mask.
9 million people die from AIDS and no one wants to wear a condom
— User says that “more and more evidence” shows that the government created novel 2009-H1N1 in a lab and released it
……