Vaccines and Related Biological Products Advisory Committee Meeting
- Nancy Cox, PhD and Alexander Klimov, PhD
- Influenza Division, National Center for Immunizations and Respiratory Diseases
- July 23, 2009
IMMUNITY TO 2009 H1N1 VIRUS RESULTING FROM PRIOR INFLUENZA INFECTION OR VACCINATION WITH SEASONAL INFLUENZA VACCINE IN DIFFERENT AGE GROUPS (NEJM submitted)
• Less than 4% of individuals born during or after 1980 exhibited preexisting, cross-reactive, neutralizing antibody titers of ≥40 to the pandemic virus, whereas 34% of individuals born prior to 1950 had titers of ≥80
• Vaccination with recent seasonal trivalent influenza vaccines (TIV), resulted in a >4-fold rises in cross-reactive antibody to the pandemic virus in
– only ~2% of children aged 6 months to 9 years,
– 12-22% of adults aged 18-64 years, and
– <5% or less of adults aged >60 years• Seasonal TIV with adjuvant induced similar cross-reactive antibody responses; no increase in cross-reacting antibody to pandemic H1N1 virus
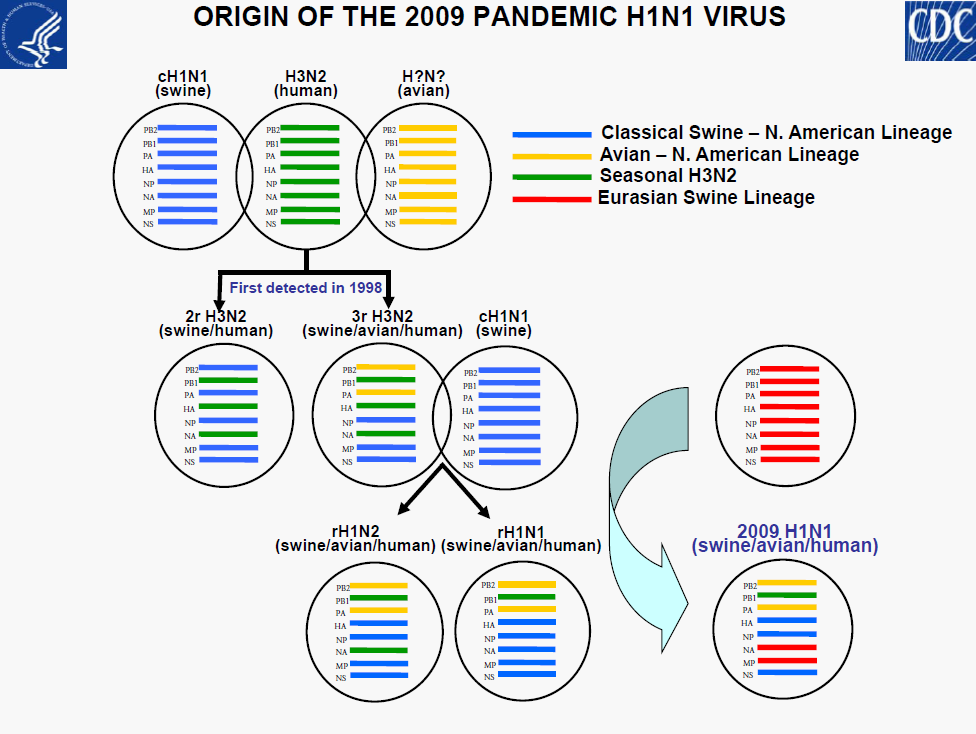
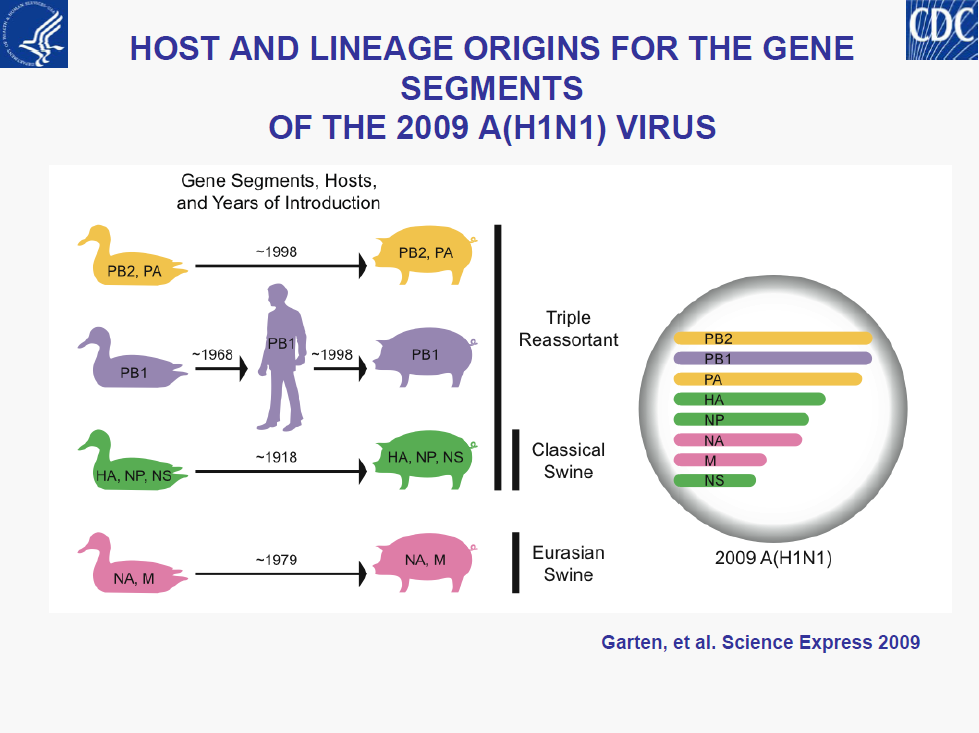
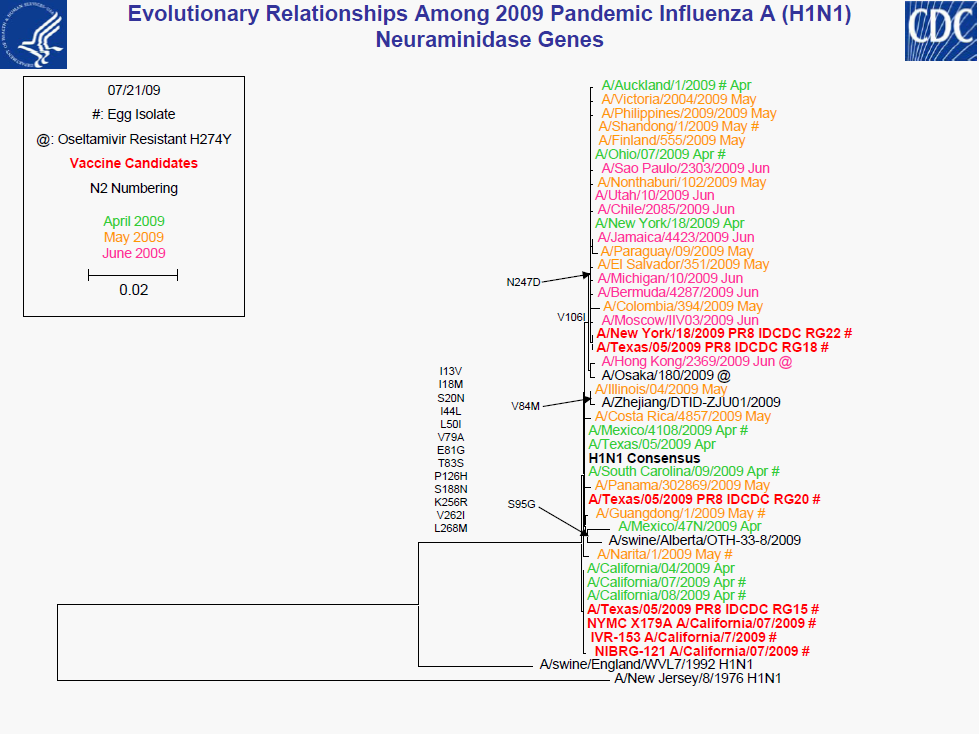
• All 2009 pandemic H1N1 viruses are antigenically similar to A/California/7/2009
• Minor genetic variability
• No evidence of reassortment with seasonal or H5N1viruses
• Resistant to M2 blockers
• Sensitive to NI (oseltamivir and zanamavir)
– Oseltamivir-resistant documented (4 of 5 after treatment or prophylaxis)
• Vaccination with contemporary seasonal influenza vaccines, with or without an adjuvant, induces little or no cross-reactive antibody to the 2009 pandemic H1N1 virus in any age group
– Individuals <30 years of age are serologically “naïve”
– A proportion of older adults appear to have pre-existing, cross-reactive antibodies
• Genetic and antigenic characterization of viruses, serologic assays, animal models, and epidemiologic assessments -all critical components for public health risk assessment
– Substantial consistency between laboratory and epidemiologic results
– Suggest novel H1N1 may not be fully adapted to humans• Epidemiologic and virologic surveillance are important for identification of future changes in
– Antigenic characteristics
– Transmission characteristics
– Severity of disease
– Antiviral resistance
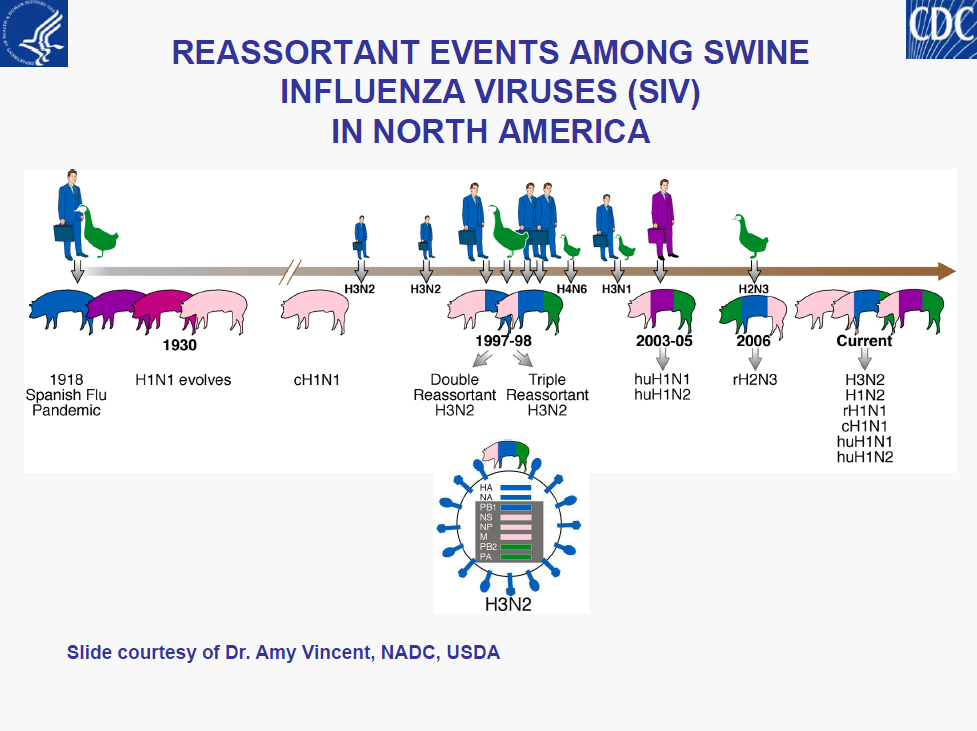
– Intensity (surge) in US cases• Limited understanding of diversity of influenza viruses in pigs globally is a major gap in pandemic preparedness
– USDA’s efforts to initiate surveillance should be supported and encouraged by public health, putting “One Health” concept into action
– Ensuring virus sharing public health, animal health, academia and industry is a key component of pandemic planning…